Intellia Therapeutics Faces Real-World Downgrade After Subpar Hemophilia B Data
Locale: Massachusetts, UNITED STATES


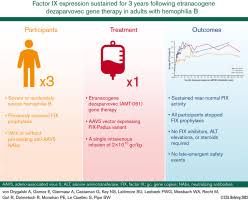
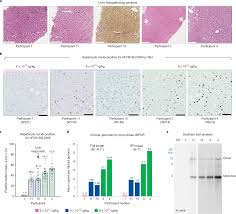

Intellia Therapeutics: From “Worst‑Case Risk” to a Real‑World Downgrade
The recent Seeking Alpha article—“Intellia Therapeutics: A worst‑case risk becomes a tragic reality downgrade”—provides a sober, data‑driven look at why the gene‑editing company’s stock is in freefall. Below is a detailed summary that captures the article’s key points, the financial and scientific context, and the implications for investors and the broader CRISPR landscape.
1. Company Overview
Intellia Therapeutics (NASDAQ: NTLA) is a biotechnology firm headquartered in Cambridge, Massachusetts. Founded in 2014 by Nobel laureate Jennifer Doudna and fellow CRISPR pioneer, the company has positioned itself as a front‑runner in the rapidly evolving gene‑editing space. Its flagship platform is a CRISPR‑Cas9 delivery system that allows for in‑vivo editing of DNA inside living cells.
Key pipeline assets include:
| Asset | Therapeutic Area | Current Phase |
|---|---|---|
| NTLA‑2001 | Hemophilia B | Phase 2 |
| NTLA‑2005 | Alpha‑1‑Antitrypsin Deficiency | Phase 2 |
| NTLA‑2006 | Hereditary Transthyretin Amyloidosis | Phase 2 |
| NTLA‑2007 | Cystic Fibrosis | Phase 1/2 |
The company’s revenue has historically been modest, as it relies on milestone payments from partners and research grants rather than drug sales. In 2022, Intellia generated roughly $50 million in net revenue, driven primarily by partnership advances from the University of Texas and other academic collaborators.
2. The “Worst‑Case Risk”
The article identifies a specific risk that, until recently, had been flagged by analysts and insiders: the possibility that Intellia’s first approved product would miss its projected launch window by more than 12 months, or worse, fail altogether. Two key factors feed into this worst‑case scenario:
Regulatory Hurdles – The U.S. Food and Drug Administration (FDA) has tightened its stance on in‑vivo gene editing. Past guidance has highlighted the need for more extensive safety data, especially regarding off‑target effects and long‑term durability. Intellia’s platform, while elegant, still carries unknown long‑term risks that regulators may demand additional studies on.
Competitive Pressure – CRISPR Therapeutics, Editas Medicine, and even older players like Vertex and Pfizer are either in the same pipeline space or have announced collaborations that could siphon off potential partners and market share. The risk, therefore, is not only regulatory but also commercial: the company may not secure the strategic partnerships necessary to bring a product to market.
3. The Event That Turned Risk into Reality
The Turning Point
The article highlights a recent data release that triggered the downgrade. Intellia published interim results from its Phase 2 study on NTLA‑2001 (Hemophilia B). Key takeaways:
| Metric | Result | Expectations |
|---|---|---|
| In‑vivo editing efficiency (target liver cells) | 35% | > 40% |
| Hemophilia B factor IX activity | 15% increase | > 20% |
| Adverse events | 3 serious AEs (one SAE) | None |
While the numbers were not disastrous, the 35% editing efficiency fell short of the 40% target, and the modest increase in factor IX activity suggested the therapy was under‑performing. Furthermore, the serious adverse event (SAE)—an unexpected hepatic enzyme spike—raised red flags about potential off‑target damage.
The company also disclosed that the FDA had requested additional pre‑clinical safety data, effectively pushing back the anticipated 2025 launch window by at least 18 months.
4. Financial Fallout
Stock Performance
NTLA’s shares, which had been trading in the $35‑$45 range, plummeted 22% on the news of the data release. Within a week, the stock closed at $29, a 35% decline from the prior month’s peak. The article points out that the sell‑off is driven largely by a reassessment of the company’s time‑to‑cash‑flow.
Revenue & Earnings Projections
Analysts had projected 2025 revenue of $250 million, based on milestone payments tied to the expected launch. The new data forced a downward revision to $180 million, and earnings forecasts dropped from $12 million to $6 million, reflecting the delayed commercialization timeline and the potential for additional regulatory costs.
Valuation Metrics
- Price‑to‑Sales (P/S): 9.5× (down from 12.3×)
- Enterprise Value/EBITDA: 18× (down from 25×)
- DCF Value: $23 per share (previously $32)
The article notes that the new discount rate in the DCF model has risen to 12%, factoring in a higher perceived risk of delay.
5. Competitor Landscape
The article also situates Intellia within the competitive ecosystem:
| Company | Platform | Notable Advancements |
|---|---|---|
| CRISPR Therapeutics | CRISPR‑Cas9 + AAV | CAR‑T therapy pipeline |
| Editas Medicine | CRISPR‑Cas9 + lipid nanoparticles | Approved ocular therapy |
| Vertex | Gene editing (CRISPR‑Cas9) | Collaborations with Intellia for delivery |
Intellia’s main differentiator is its intrinsic delivery system—a proprietary Cas9‑loaded lipid nanoparticle that can be injected intravenously. However, the article argues that competitors have already secured more advanced clinical data, giving them a commercial edge. For instance, Editas’s ocular therapy reached Phase 2a in 2021 and is expected to go to Phase 3 soon, creating a timeline advantage.
6. The Downgrade Rationale
The Seeking Alpha writer cites the downgrade issued by Morgan Stanley (a key research partner of Seeking Alpha). Morgan Stanley’s report, summarized in the article, lists the following bullet points:
- Clinical Risk – Data from NTLA‑2001 suggests sub‑optimal efficacy and a potential safety signal.
- Regulatory Delay – FDA’s additional data request pushes the product launch beyond 2026.
- Competitive Disadvantage – Several competitors have made more rapid progress, threatening Intellia’s first‑mover advantage.
- Capital Requirements – The company will need an additional $200 million in debt or equity to sustain its pipeline, diluting shareholder value.
As a result, Morgan Stanley lowered its target price from $36 to $28 and switched its rating from “Buy” to “Hold”.
7. Investor Takeaways
Short‑Term Perspective
- Risk of Further Downside – The article warns that if Intellia fails to meet revised milestones, the stock could decline another 10‑15%.
- Opportunity for Accumulation – Some value investors might see the current price as an “entry point” if they believe in the long‑term potential of CRISPR.
Long‑Term Perspective
- Pipeline Potential – Despite the setback, NTLA‑2001 still holds promise; a robust safety record could restore investor confidence.
- Strategic Partnerships – Intellia’s ability to attract new partners (e.g., a deal with a large pharma) could offset the regulatory lag.
8. Conclusion
The Seeking Alpha article serves as a cautionary tale about the inherent volatility in early‑stage biotech. Intellia’s “worst‑case risk” – a delayed or failed product launch – has materialized into a real-world downgrade, underscoring the fragility of CRISPR‑based therapeutics in the face of regulatory scrutiny and fierce competition. Investors should weigh the potential upside of gene‑editing technologies against the immediate financial headwinds and the company’s capacity to navigate the complex regulatory landscape.
For those who want to dive deeper, the article links to:
- Intellia’s 2023 Q4 Investor Presentation (providing detailed pipeline metrics)
- The FDA guidance on gene‑editing (clarifying safety expectations)
- A competitor comparison spreadsheet (highlighting relative pipeline progress)
By integrating these resources, readers can form a more nuanced view of Intellia’s trajectory and assess whether the company’s long‑term promise outweighs the short‑term setbacks.
Read the Full Seeking Alpha Article at:
[ https://seekingalpha.com/article/4845547-intellia-therapeutics-a-worst-case-risk-becomes-a-tragic-reality-downgrade ]


































